Bringing a biologic therapy from concept to scalable manufacturing requires more than just expertise—it demands precision, customization, and a commitment to uncompromising quality.
We offer a full suite of biologics development services that can be integrated into a seamless workflow or accessed individually, depending on your project needs. Whether you require end-to-end support or a specific service, our expertise ensures optimal results at every stage of your biopharmaceutical program.
Why choose us
Over 15 years of biologics development experience, delivering reliable solutions and driving advancements in the field.
Custom strategies designed to match your molecule, therapeutic goals, and regulatory pathway.
ISO 9001:2015 certified, ensuring quality-first solutions trusted by our clients.
We provide clear timelines, open communication, and full project visibility at every stage.
From cell line development to process optimization and bioanalytics, our expert-driven approach ensures a seamless transition from early-stage research over scalable manufacturing to clinical analytics—all while maintaining the highest scientific and regulatory standards.
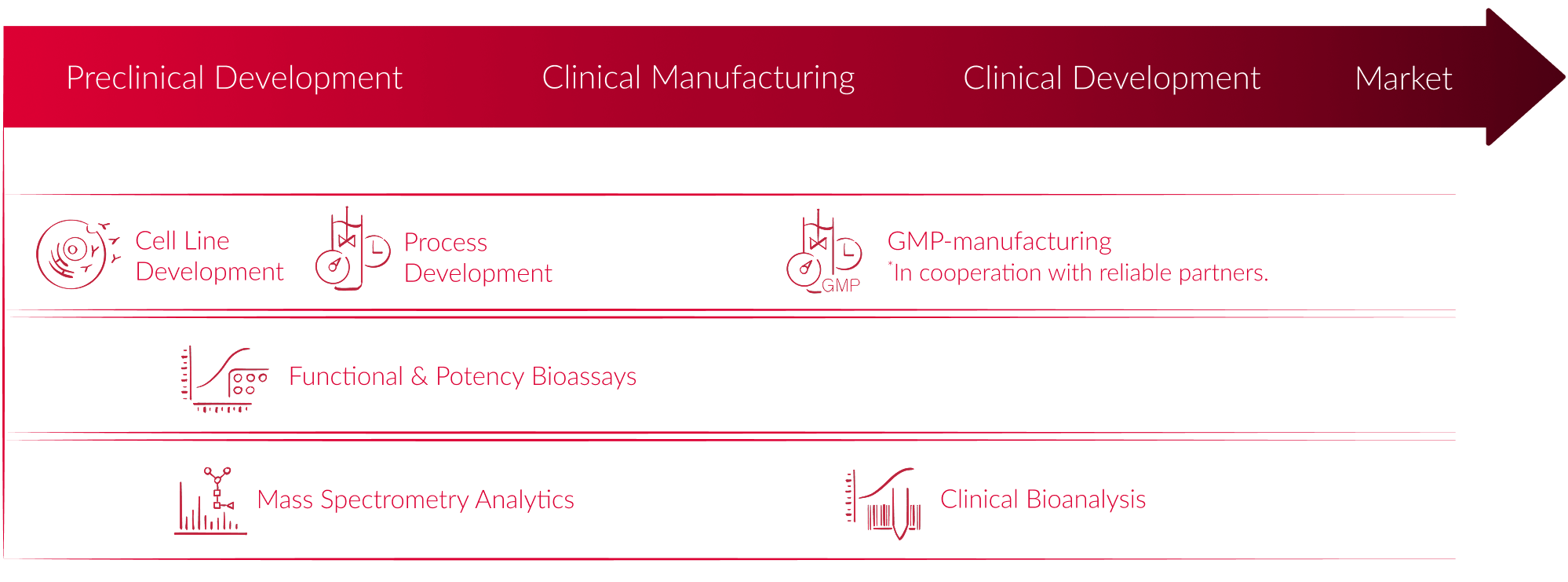
A strong foundation begins with the right cell line. Our proprietary CHOnamite® and human GlycoExpress® (GEX®) platforms deliver stable, high-yield cell lines, accelerating your pipeline with confidence.
With deep expertise in both upstream and downstream processes, we help you achieve consistent product quality, structural integrity, and seamless transition to GMP manufacturing. Our integrated analytical and bioassay capabilities support in-depth characterization at every stage.
Our broad portfolio of analytical assays, combined with deep expertise in bioassay development and validation, delivers critical insights to support every stage of your drug development, from research to clinical phases.
We use advanced mass spectrometry for the assessment of critical quality attributes, such as glycan profiling, at every stage of development.
Our bioanalytical services include GCLP-compliant ligand binding assays, cell-based assays, and hybrid LC-MS/MS methods, tailored to support PK, PD, and immunogenicity studies. With proven expertise in method development, validation, and sample analysis, we help you navigate clinical bioanalysis with precision and confidence.
To support a smooth path from development to manufacturing, we offer our customers a seamless transition to one of our trusted GMP partners. With a well-established global network, we coordinate closely to ensure continuity, transparency, and tailored support every step of the way.
Bringing biologics from research to scalable production comes with scientific and technical challenges. At FyoniBio, we are the partner you can trust for a successful biologics project. With our expertise, we overcome bottlenecks with targeted solutions, ensuring reliability and regulatory success.
Solutions
01
Challenge
Our Solution
02
Challenge
Our Solution
03
Challenge
Our Solution
04
Challenge
Our Solution
05
Challenge
Our Solution
What We Deliver

Erica Kerkvliet, VP R&D
Pharming Group N.V.

Pierre Dönnes, Co-founder & VP Neoantigen Discovery
Strike Pharma AB
We combine scientific expertise and creative thinking to empower our customers to bring therapeutic breakthroughs to market. Whether you're a biotech startup, mid-sized biopharma, or a pharmaceutical company, our customized solutions approach ensures your success.
Connect with our experts today to explore how our tailored services can support your biologics development journey.
Our expertise

“Each molecule has its own needs—and by combining tailored cell line development with a versatile set of host systems, we lay the groundwork for a robust production process. With added strength in upstream development and a close eye on quality parameters like glycosylation, we build commercially viable processes ready for GMP manufacturing.”

“Our tightly interwoven expertise in protein purification, formulation, and biochemical analysis forms the backbone of our team. With experience across all protein types, we develop processes and methods that meet even the most specific requirements—always with an eye on future GMP compliance.”

“Two decades of experience in glycoprotein analytics and our reliable UPLC/QTOF-MS systems enable us to deliver conclusive, meaningful insights—especially where others fall short. Just as important: we communicate openly and clearly, so you always know what the data means and what to do next.”
Our experienced team is here to provide the analytical support and insights
you need to move your program forward efficiently, confidently, and without delays.