Expression of protein-based biopharmaceuticals with human characteristics requires a biological system close to its origin.
To address this need, we developed GlycoExpress® (GEX®)—a human cell line platform tailored for the production of glyco-optimized biotherapeutics.
Our well-established, extensively characterized human expression platform engineered for the screening and production of glyco-optimized biotherapeutics with authentic post-translational modifications. This versatile toolbox of human cell lines supports the development of complex glycoproteins with high yield, scalability, and human-like quality attributes.
Solutions
The GlycoExpress (GEX) platform has been successfully used in a wide range of biopharmaceutical programs, including:
01
Application
02
Application
03
Application
04
Application
05
Application
06
Application
Solutions
The GlycoExpress (GEX) platform has been successfully used in a wide range of biopharmaceutical programs, including:
Complex antibody isotypes (e.g., IgA)
Difficult-to-express and complex glycosylated proteins
Blood factors (e.g., FVII)
Protein hormones (e.g., FSH, HCG)
Fusion proteins with extended serum half-life
Enzymes (e.g., for enzyme replacement therapy)
Why choose us
For proteins where glycosylation impacts function, stability, immunogenicity, or half-life, using a biologically relevant human host system is critical.
01
BENEFIT
Homogeneous, non-immunogenic glycan profiles for complex mammalian proteins
02
BENEFIT
Tailored sialylation, fucosylation, and mannose-6-phosphate levels
03
BENEFIT
Reduces the risk of immunogenicity
04
BENEFIT
Achieves up to 30 g/L in perfusion for IgGs; adaptable to large-scale bioreactors
05
BENEFIT
Stable glycosylation and minimized batch-to-batch variation
06
BENEFIT
Over 40 GMP production runs; globally approved by agencies including FDA and EMA
Why choose us
For proteins where glycosylation impacts function, stability, immunogenicity, or half-life, using a biologically relevant human host system is critical.
Homogeneous, non-immunogenic glycan profiles for complex mammalian proteins
Tailored sialylation, fucosylation, and mannose-6-phosphate levels
Reduces the risk of immunogenicity
Achieves up to 30 g/L in perfusion for IgGs; adaptable to large-scale bioreactors
Stable glycosylation and minimized batch-to-batch variation
Over 40 GMP production runs; globally approved by agencies including FDA and EMA
Every therapeutic protein has unique glycosylation requirements. Our GEX® platform offers a modular set of human cell lines that allow you to precisely adjust critical glycan attributes—supporting optimal bioactivity, reduced immunogenicity, and extended serum half-life.
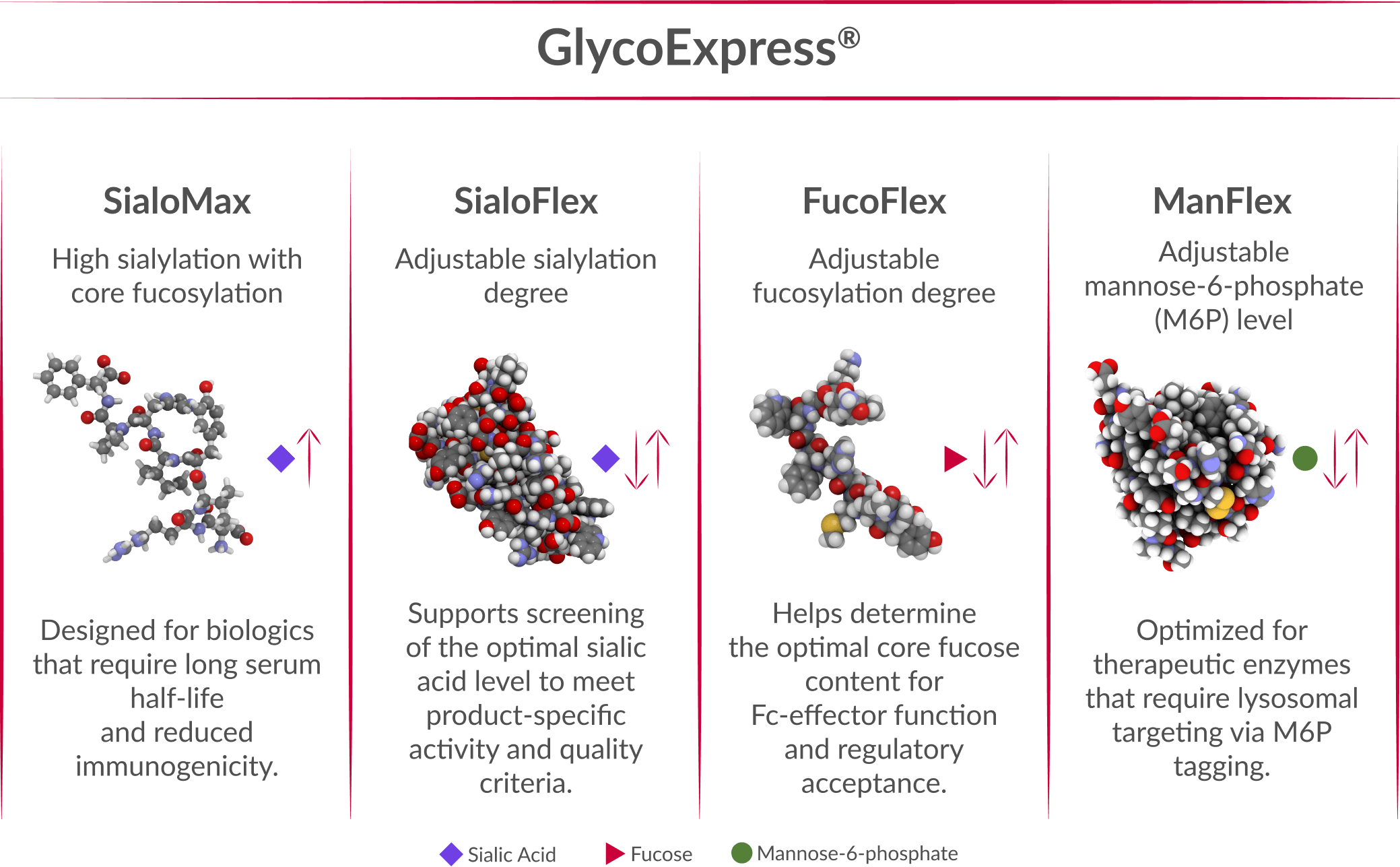
With GEX®, you can precisely modify and control post-translational modifications to meet your therapeutic goals. New glycoengineered GEX® variants are continuously developed to support even the most challenging projects.
GEX®, combined with our proven bioprocessing platforms, enables stable, high-quality biomanufacturing tailored for commercial success. Its consistent product quality and low aggregation levels throughout processing help reduce downstream complexity and cost, making it an efficient and scalable solution.
With over 40 GMP production runs completed across multiple projects, GEX® has demonstrated exceptional reproducibility—regardless of batch size, scale, or production site. This includes bioreactor volumes of up to 1000 L, ensuring flexibility, reliability, and confidence in every manufacturing phase.
GEX®-derived glycoproteins have been approved by regulatory authorities around the world (incl. FDA, PEI, and BfArM) for use in clinical trials in humans. No viruses, viral particles, or reverse transcriptase activity have been detected in any of the ICH-compliant safety test panels conducted to date.
Regulatory bodies including the FDA and EMA have confirmed that GEX® cell line characterization meets appropriate guidance, supporting an unproblematic market entry path.
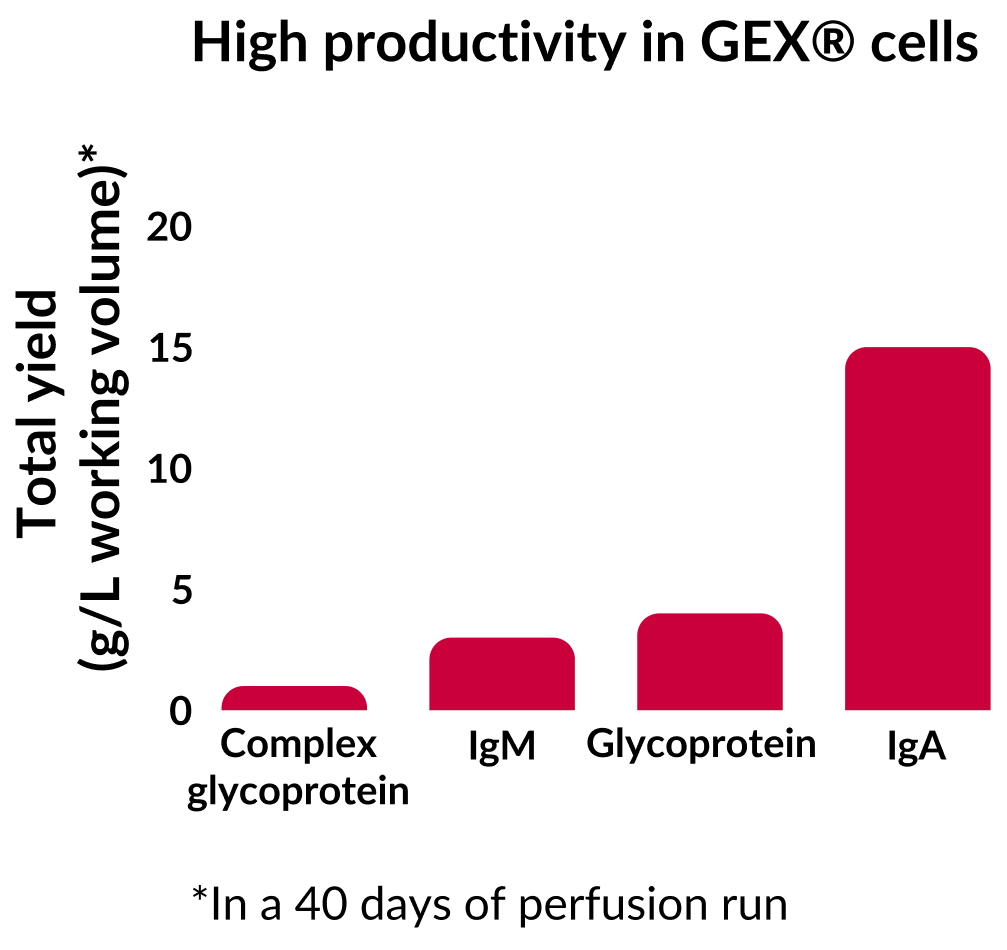
In perfusion processes over 30-40 days we produced 30 g/L reactor volume for IgG, 15 g/L reactor volume for IgA, 3 g/L reactor volume for IgM, and 2 g/L reactor volume for a blood factor protein.
For small scales we offer shake flask, spinner flask, and up to 5L bioreactor batch cultivation. If more product is asked for, small scale mock perfusion or 1L perfusion will be employed. Alternative production modes including intensified fed-batch processes can be implemented.
For GEX® cells in-house developed cultivation and process media are available which can be ordered by the customer. Alternatively, commercially available media are suitable for GEX® cells but require a pre-adaptation of 3-4 weeks.
FyoniBio offers comprehensive packages for cell line, process, and analytical development. Our integrated approach ensures the expertise, quality, and speed needed for a smooth transition to GMP manufacturing and clinical development.