Immunogenicity Testing
Assessment of the ADA Response for a Wide Range of Biotherapeutics
FyoniBio has many years of experience in immunogenicity testing services including assay development, validation and bioanalysis in the course of clinical trials. All bioanalytical studies are performed according to the current ICH, FDA and EMA guidelines in our ISO 9001 certified and GCLP compliant laboratories. Get in touch with us, we will be happy to support you throughout your clinical development program.
What is immunogenicity and why it is important?
Biotherapeutics from small oligonucleotides to large proteins can elicit immune responses when applied in animals or humans. The immune response may result in the formation of anti-drug antibodies (ADA). The probability to develop ADAs depends on the application route and design of the biotherapeutic. It ranges from a few patients to high incidences in the mid double-digit range.
Binding of ADA to the drug results in clinical responses that range from no symptoms to severe side effects, and may even be health- or life-threatening. The presence of ADA may alter the pharmacokinetics, may neutralize the drug’s activity, and affect the mode of action, may support hypersensitivity or autoimmunity reactions, or may cross-react with endogenous counterparts of the drug.

In order to evaluate the safety, efficacy and pharmacokinetics of a biotherapeutic drug, its immunogenic potential has to be assessed during preclinical and clinical development. Immunogenicity testing means, that it is examined whether or not anti-drug antibodies are detectable in the blood after drug application. In preclinical animal studies the occurrence of ADA is mainly analyzed to evaluate any effects on the bioavailability of the drug. In humans, immunogenicity testing as a key safety aspect is included in clinical trials from phase I onwards.
Immunogenicity Analysis
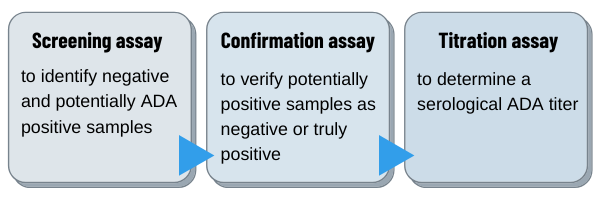
Samples are usually analyzed in a three-tier approach using
i) a screening assay to identify negative and potentially positive samples
ii) a confirmation assay to verify the potentially positive samples as negative or positive
iii) a titration assay to determine a serological titer.
Major challenges which have to be solved during ADA assay development are the availability of a suitable positive control, sensitivity, drug tolerance, matrix effects including pre-existing antibodies, and the determination of the assay cut point. To generate an appropriate positive control we recommend our preferred partner company BioGenes who have plenty of experience in generating suitable antibodies against almost any antigen.
Clinical samples are analyzed by using all three assays of the approach whereas preclinical samples are typically only analyzed using the screening and confirmation assay.
Immunogenicity Assay Design
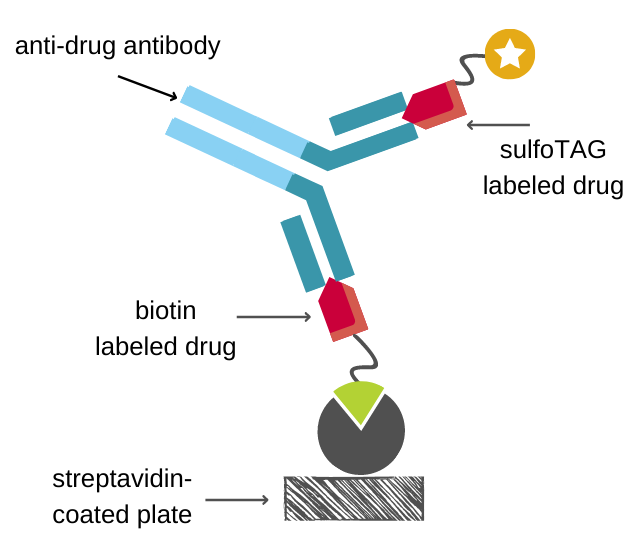
At FyoniBio, anti-drug antibodies are detected using an electrochemiluminescence (ECL) assay in a homogeneous bridging format. Samples are diluted at the minimally required dilution to overcome potential matrix effects. Diluted samples are subjected to an acid dissociation step to dissolve complexes of anti-drug antibodies and the drug, thereby improving greatly the drug tolerance. Next, samples are neutralized and simultaneously incubated with biotinylated and sulfoTAG-labelled drug to start immune complexes formation. Subsequently, the solution is transferred to a blocked streptavidin coated 96-well plate. Unbound proteins are removed by washing and ECL is measured on a MESO QuickPlex SQ 120 (MSD Inc.). The measured light signal is proportional to the concentration of anti-drug antibodies in the sample.
Immunogenicity Assay Evaluation
Evaluation of the measured signal is done qualitatively, using assay specific cut-points established during validation. As a result of the immunogenicity assay, a sample’s signal may be above or below the cut point, classifying the sample as positive or negative. Screening-positive samples are subsequently analyzed in a confirmation assay in absence and presence of the drug. Sufficient ADA signal inhibition by the drug verifies a sample as truly positive. Finally, confirmed positive samples are analyzed in the semi-quantitative titration assay where the Log2 titer of a sample is determined from a 2-fold serial dilution.
For positive samples, further characterization of the isotype and the neutralizing activity of the ADA might be performed.
Watch our latest videos explaining the basics of immunogenicity related to biotherapeutic drug application.
Need more information about our Immunogenicity Testing Services?
Frequently Asked Questions (FAQ) for Immunogenicity Analysis
Any biological drug, from small oligonucleotides and peptides to large therapeutic proteins such as antibodies, may elicit an immune response when applied to animals or humans. However, the risk of unwanted immune responses depends on size, structure, and application route, among others. Generally, immunogenicity testing is required for all biological drugs, based on a risk assessment.
Assays used for the immunogenicity assessment of patient samples have to be validated for their intended purpose. Fully validated assays have to be used within pivotal studies required for marketing authorization application.
The assay development time depends on the project and may be affected by availability and quality aspects of the critical reagents, matrix effects and drug tolerance issues. Typically, a new assay is established and prepared for validation within 3 to 4 months. The experimental phase of assay validation takes about 2 to 4 weeks and the subsequent reporting including QC/QA will be accomplished in another 4 weeks. We recommend to get in touch with us early to discuss the requirements of your project and to avoid potential delays.
For more relevant information and additional FAQs check also the FAQ section in our resource center.
Immunogenicity Assessment at FyoniBio – Related Content
Have a look at the relevant guidelines describing the principles of bioanalytical method validation.
Immunogenicity Testing – Downloads
Application note: How to perform immunogenicity testing

