Pharmacokinetic Analysis
Assessment of the Drug Levels in Patient Samples for a Wide Range of Biotherapeutics
FyoniBio has a long history of working in the field of bioanalysis in clinical trials. It has accumulated many years of proven experience and expertise in assay design, development, validation and its application to the analysis of clinical samples. We can perform pharmacokinetic analysis of clinincal samples according to the current ICH, FDA and EMA guidelines in our ISO 9001 certified and GCLP compliant laboratories. FyoniBio supports pharmacokinetic studies from anti-drug antibody generation, over assay validation to the analysis of study samples, whereby the generation of antibodies is performed by our partner company BioGenes.
Get in touch with us, we will be happy to support you throughout your clinical development program.
What is pharmacokinetics and why it is important?
Pharmacokinetics (PK) investigates how the body deals with the drug, in contrast to pharmacodynamics (PD) which examines how the drug deals with the body. PK describes the processes that define the time course of a drug and its major metabolite concentrations in serum, plasma or other biological fluids in order to obtain information on absorption, distribution, metabolism and elimination (ADME) and therefore indicates the drug exposure of the patient. Understanding PK/PD is a key element of drug development and PK assessment is therefore included in preclinical and clinical studies. PK analyses are used to characterize drug exposure and bioavailability, to predict dosage requirements and assess dosage changes, to describe individual subject variability and to establish safety margins.
In clinical studies, PK assessment is primarily required for dose escalation and dose finding studies. The PK of a drug depends on patient-related functions, such as individual physiological characteristics, as well as on the drug’s biochemical properties.

Our Core Competencies in Pharmacokinetic Analysis
- PK assay design and development (including antibody generation with our partner company BioGenes GmbH)
- PK assay validation
- PK sample analysis including incurred sample reanalysis
- Non-compartmental PK evaluation
- Sample management and storage
- Consultancy in clinical study design with respect to analytical program
Design and Development of PK Assays
Our experienced analytical scientists consider the biochemical properties of the drug, the requirements for its clinical application and the customer needs to develop an appropriate PK assay. Therefore, a number of techniques for the development of the PK assay are available at our facility, including mass spectrometric (MS) methods and ligand binding assays (LBAs). For LBAs we use either an enzyme-linked immunosorbent assay (ELISA) or an electrochemiluminescence immunoassay (ECLIA) on the MSD platform.
Immunoassay Setup in Sandwich Format
The most common format of LBA-based PK assays is the sandwich format. In principle, the analyte (therapeutic protein or drug) is “sandwiched” between the capture antibody, antigen, or soluble receptor (which is immobilized on a surface) and the detection antibody, antigen, or soluble receptor. It is particularly useful for complex samples, such as blood samples and we mostly perform it in three versions which are described in the following.
Antibody Capture Assay
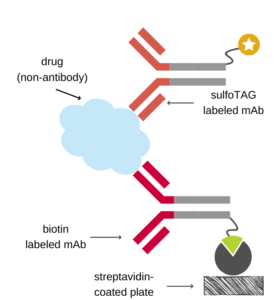
The antibody capture assay can be applied for any biotherapeutic drugs. Prerequisite to perform antibody capturing is the availability of a pair of antibodies directed against the analyte. In case of antibody drugs an anti-idiotypic (ID) antibody is immobilized on the plate and is used to capture the drug, while a second antibody is labeled with an appropriate tag and is used to detect the drug. The detection antibody could be the same anti-ID antibody, an anti-Fc antibody, or alternatively, antibody-binding proteins, such as protein A/G. For non-antibody drugs one needs a pair of matched anti-drug antibodies for capturing and detection. It is the optimal format to get rid of matrix effects, but it requires the generation of at least one anti-drug antibody. Contact our partner company to get a suitable antibody or antibody pair.
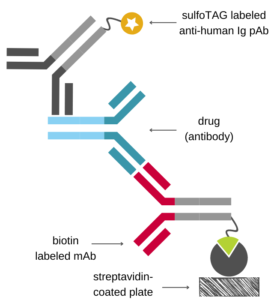
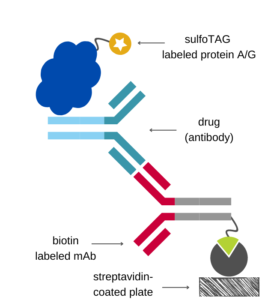
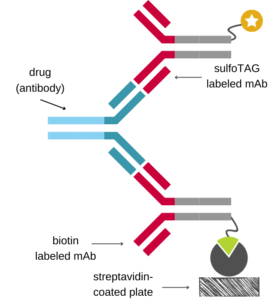
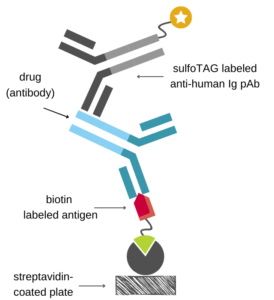
Antigen Capture Assay (for Antibody Drugs)
Ιn the antigen capture format, the immobilized antigen is used to capture the drug onto the assay plate which is then detected using a species-specific anti-Fc antibody labeled with an appropriate tag.
If the antigen is available, this setup is relatively easy to implement, since it only requires a commercially available detection antibody. However, components of the complex matrix of blood samples (e.g., endogenous antigen) may interfere with the antigen-antibody binding.
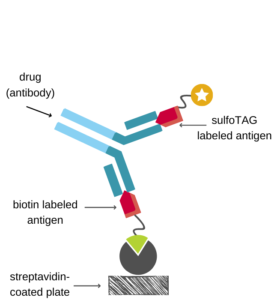
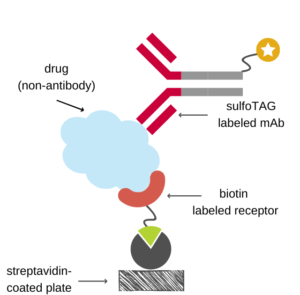
Soluble Receptor Capture Assay
Many therapeutic proteins in drug development bind to receptors with high affinity that either circulate in the blood or are integral part of cellular membranes. If these receptor proteins are available in a soluble form, a soluble receptor capture format could be the assay format of choice, if an appropriate antibody is missing.
The immobilized soluble receptor is used to capture the drug onto the assay plate which is then detected using a drug specific detection antibody. As described for the antigen capture assay, the complex matrix of blood samples has the potential to impede assay development.
In all formats the capturing protein can be directly coated onto a microtiter plate or linked via the streptavidin-biotin system. In many cases immobilization via streptavidin-biotin improves assay performance. However, biotinylation of the capturing protein may also affect the binding to the drug, especially if the capturing protein is small.
Which assay format is the most appropriate for a specific drug and application?
Our scientists will select the most appropriate assay format for each project depending on the customer needs and the specific pros and cons of each format. The table below lists the advantages and disadvantages of each format.
| Assay Format | Advantages | Disadvantages |
|---|---|---|
| Antibody Capture Format | Low risk for interference with serum components | An antigen-specific antibody needs to be available. In case of non-antibody drugs, the size of the drug needs to allow two independent antibody binding sites. |
| Antigen Capture Format | No need to generate new antibodies, if the antigen is available | Antigen may be of limited source; interference of antigen-antibody binding by serum components (e.g., endogenous antigen or soluble receptors) |
| Soluble Receptor Capture Format | No need to generate new antibodies, if the soluble receptor is available | Soluble receptor may be of limited source; interference of soluble receptor-drug binding by serum components (e.g., endogenous soluble receptors) |
Are you searching for an appropriate PK assay for a multi-functional drug?
Many therapeutic proteins currently in drug development are characterized by more than one functional domain, like fusion proteins, ADCs, or bispecifics. To select an appropriate PK assay format for those drugs, one need to consider each functional domain to ensure that the measured drug concentrations cover the whole protein with all its functional domains.
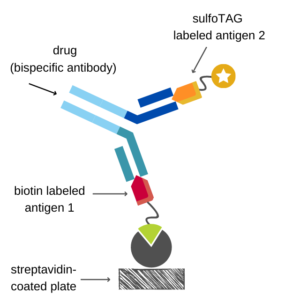
In our hands, the formats described above can be adapted for therapeutic proteins with more than one functional domain. For example, one functional domain is used for immobilization and the second for detection. That could be realized in a bridging format as illustrated in the figure for a bispecific antibody. The binding reactions of a bridging assay can be performed in one step in solution.
Contact us to find the most appropriate assay format for your therapeutic protein.
Analysis of Free, Partially Free and Total Drug
In PK and toxicology studies it is often essential to estimate the different forms of the drug present in biological fluids i.e., free or partially free, bound and total drug. The level of the free drug reflects the availability of the active drug, while the levels of the bound and the total drug are used by toxicologists to estimate the safety of the drug.
Definitions of free, partially free, bound and total drug using a therapeutic antibody as an exemplary drug are given in the table below.
| Form of Drug | Definition | |
|---|---|---|
| Free antibody drug |  | Therapeutic antibody that has both antigen binding sites free |
| Partially free antibody drug | 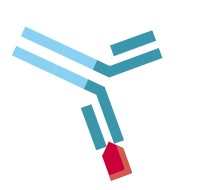 | Therapeutic antibody that has one antigen binding site free |
| Bound antibody drug | 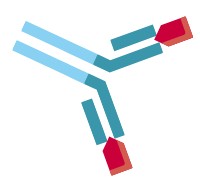 | Therapeutic antibody that has at least one (partially) or both (free) antigen binding sites free |
| Total antibody drug | Sum of free, partially free and bound drug | |
Analysis of each drug form is performed with the same immunoassay formats described above. The table below summarizes appropriate detection methods including capturing and detection reagents when using a therapeutic antibody as an example.
| Form of drug | ELISA method |
|---|---|
| Free and partially free antibody drug | Antigen capture assay with antigen for capture and anti-Fc antibody for detection Antibody capture assay with competitive1 anti-ID antibody for capture and competitive or non-competitive2 anti-ID antibody for detection |
| Total antibody drug | Antibody capture assay with non-competitive anti-ID antibody for capture and non-competitive anti-ID antibody for detection |
| Bound antibody drug | Calculated as difference between total and free/ partially free antibody drug |
1) Competitive anti-ID antibodies can only bind to the antibody drug in the absence of the antigen; 2) Non-competitive anti-ID antibodies bind to the antibody drug independently of the antigen
Assay Validation
The validation of an assays ensures that the test results are precise, accurate and reliable, and that the analytical method is fit for the intended purpose.
Learn more about assay validation.
Need more information on the development of PK assays?
Get in touch and discuss with our scientists the requirements of your specific project and learn how FyoniBio may support your drug development program.
Frequently Asked Questions (FAQ) for PK Analysis
Pharmacokinetic is sometimes described as what the patient’s body does to the drug. It comprises the processes and time course of liberation, adsorption, distribution, metabolism and excretion of the drug.
PK data can be used to link the drug exposure to the observed efficacy and safety. Knowledge of drug levels helps in choosing the appropriate application route, dosage, and schedule of a drug and may furthermore indicate immunogenicity effects. The assessment of a drug’s PK is therefore a very important tool during pre-clinical and clinical drug development.
For establishment and validation of our bioanalytical methods we apply the current international bioanalytical guidelines of ICH, FDA and EMA (see also guidelines to bioanalytical method validation).
– ICH Guideline Q2(R1): Validation of Analytical Procedures, Nov 2005
– FDA Guideline Bioanalytical Method Validation, May 2018
– EMA Guideline Bioanalytical Method Validation, Feb 2012
For more relevant information and additional FAQs check also the FAQ section in our resource center.
