Identify the Best-Fitting Host Cell within a Feasibility Study
Start your Cell Line Development right: Explore the Potential of our Diverse Cell Platforms
Do you have problems with your recent protein project? Try our GlycoExpress® and CHOnamite® cell lines to improve the performance of your project. FyoniBio offers a feasibility study at reasonable costs to explore your protein’s potential in multiple host cell platforms fast and efficient. A feasibility study is therefore the ideal starting point for your cell line development journey.
What makes selecting the optimal host cell so important?
- Productivities and quality characteristics of a biotherapeutic are highly dependent on the selected host cell line. A smart and early selection of a well-fitting cell line helps to obtain desired product characteristics and commercially viable production processes.
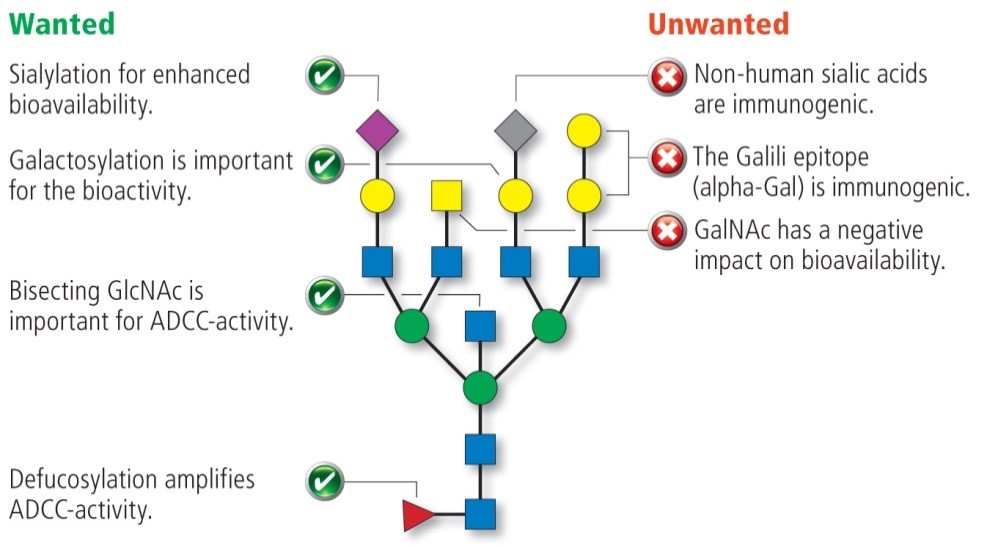
- Glycosylation differs from one parental cell to another and is qualitatively different in human and rodent cell lines. A number of key properties of a biotherapeutic such as bioactivity, solubility, stability, serum half-life and safety are influenced by its glycosylation pattern. Therefore, modification and control of glycosylation and other posttranslational modifications (PTM) is considered a key feature for the development of a biotherapeutic drug.
- Expression machineries in different parental cell lines may favor some proteins structures and PTM´s over others. We found that our human cell host GEX® works better on difficult-to-express proteins such as complex glycoproteins or alternative antibody- formats compared to CHO based systems.
- Proteins sensitive to degradation may benefit from a production process like perfusion. Parental cell hosts, however, may be more suitable for fed-batch than perfusion. Luckily, both GEX® and CHOnamite® can be used in fed-batch or perfusion mode tailored to individual protein and manufacturing process requirements!
Feasibility Study in GlycoExpress® and CHOnamite® Host Cells
Because different products need specific quality and glycosylation characteristics for their optimal activity, a set of GlycoExpress® (GEX®) and CHOnamite® cell lines with the following attributes are available:
- mAbExpress and mAbExpressF- for optimized glycosylation of antibodies, including high galactosylation, bisGlcNAc, sialylation, as well as high degree of fucosylation (mAbExpress) or lacking core-fucose (mAbExpressF-) for ADCC enhancement
- SialoMax used for products where high sialylation and high core fucosylation is required
- SialoFlex and FucoFlex for products where a specific degree of sialylation (SialoFlex) or fucosylation (FucoFlex) is needed, e.g. for screening of the optimal content of sialic acid or fucose in functional bioassays or to comply with required quality acceptance criteria
- CHOnamite® as our in-house developed, diverse CHO host platform for high yield expression of simple and complex mammalian proteins and biosimilar development.
Key Facts about GlycoExpress® and CHOnamite® Host Cells
GlycoExpress® (GEX®) are human cells suitable for the production of authentic human glycosylated proteins without immunogenic non-human carbohydrate residues.
Learn more about the the GlycoExpress® Platform for Human Cell Line Services.
CHOnamite® is our in-house developed CHO platform originated from CHO-K1 and CHO-DG44 cells. The technology enables high yield production of antibodies and complex proteins.
Successful Case Studies were performed with antibodies of different isotypes and formats including bispecifics, difficult-to-express proteins, blood factors, hormones and enzymes.
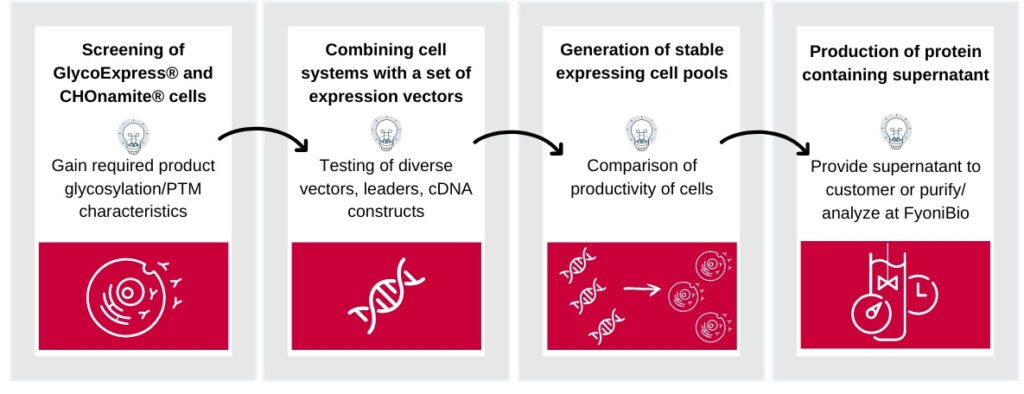
Procedure of a Cell Line Feasibility Study
Finding a suitable host cell for each individual biotherapeutic is critical for successful product manufacturing. FyoniBio offers a 4 step approach to evaluate the potential of your biotherapeutic across multiple expression platforms.
Combining Cell Platforms with a Set of Expression Vectors
FyoniBio offers tailor-made expression vectors for protein production in GEX® and CHOnamite® cells. We operate with selected vendors for the synthesis of sequence optimized DNA. A set of expression vectors is available to analyze the following parameters and find the best solution for each project:
- Leader sequences
- Enhancer/promotor sequences
- cDNA versus genomic DNA
Generation of Stable Host Cell Pools
Stable transfection is performed by highly efficient electroporation and stable cell pools are generated by applying the appropriate selection pressure. Production titers are determined by standard methods (e.g., ELISA, Octet). We also involve our analytical services and bioassay team, who apply protein-specific analytical methods or develop assays from scratch if necessary. Initial selection of best producing pools is based on the obtained productivity data.
Production of Supernatant for Further Quality Assessment
Depending on the desired amount of protein, supernatant is produced from stable cell pools in spinner/ shake flask cultures (up to 2L) or in lab scale batch processes (up to 5L). Quality control of produced supernatant or purified protein is performed directly at FyoniBio’s analytical services & bioassays department based on customer requirements (e.g., PTMs, aggregates). Alternatively, supernatant is provided to the customer for analysis.
Explore how the potential of your biotherapeutic can be evaluated across multiple cell line platforms in our customized feasibility study. Watch our explaining video.
Need more information about our feasibility study to identify the best host cell?
If you are interested in performing a feasibility study using GlycoExpress® and CHOnamite® at reasonable costs, get in touch with us.
Frequently Asked Questions (FAQ) for Host Cell Feasibility Studies
We often see, that different products drastically differ in productivity when derived from different host cells. Sometimes GEX cells show a 10-fold higher productivity than CHO and sometimes vice versa. Furthermore, different products need different quality and glycosylation characteristics for their optimal activity Therefore, a pre-evaluation of cell lines can be an efficient way to directly find the optimal production host. In combination with a diversity of expression vectors including different regulatory sequences, promotors and leader peptides, best possible productivities, secretion, and product activity can be obtained. This provides the basis for a successful clone development.
In a feasibility study an evaluation of GlycoExpress® and CHOnamite® cell lines is performed to find the optimal solution for the production of each individual protein within 2-3 month turnaround time. Thereby different cell lines (WT and glyco-engineered) can be combined with a set of expressing vectors to optimize productivity, protein secretion and activity. In this way, stable pools are generated and compared for individual and customized parameters (productivity, activity, quality). A number of pools can be further subjected to supernatant production in batch cultures (up to 5L). Supernatant can be directly analyzed at FyoniBio based on customer requirements or shipped to the customer.
For feasibility testing you mainly chose from GlycoExpress®, a human cell line platform, and CHOnamite®, a set of in-house developed CHO cell lines. The GlycoExpress® platform includes a set of different human cell lines glycoengineered to address adjustment of protein sialylation, fucosylation, and galactosylation. CHOnamite® encompasses cell lines originating from CHO-K1 or CHO-DG44. In CHO, the use of different host cells allows a more diverse approach, which is particularly useful in the development of biosimilars.
Starting from preparation of stable cell pools up to production of larger amounts of protein containing supernatant as well as purification and analysis of the product a period of around 3 months is required. Exact time lines can be prepared and discussed after understanding your individual project requirements.
Host Cell Feasibility Study – Related Content
Learn more about the GlycoExpress® Platform for Human Cell Line Services.
Explore how our USP and DSP process development services can be integrated in your cell line development journey.




